Avacta retains a significant set of oncology assets. But this, by itself, is not enough to be successful in the biotech space.
Before we get started, I want to make clear that this is not an article designed to rub salt in the wound. If you are feeling betrayed, hurt, financially violated, or otherwise — it may be best to close the page and come back to this in a few weeks.
I do not enjoy seeing investors lose money, almost all of whom invested in good faith.
But if you have lost funds on Avacta (paper or real), it’s worth at least getting some important tuition out of the experience. These are just personal views; but they will likely resonate.
Let’s dive in.
The warning signs
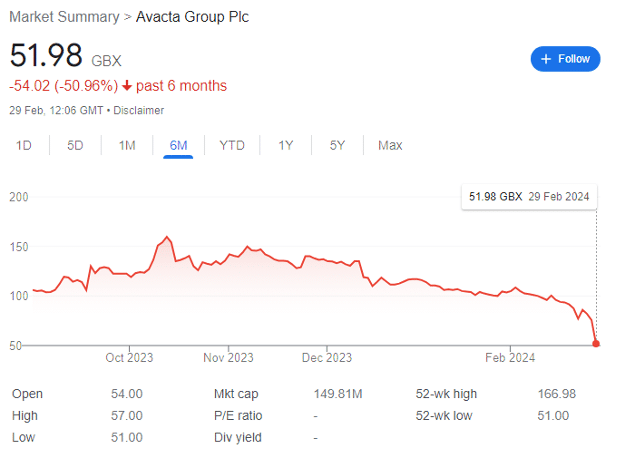
There’s the first one. When the share price falls by more than 50% from a high of circa 160p in October 2023 to less than 80p a few short months later — even though the flagship asset is progressing — then you have to know something’s up.
For clarity, this rule applies to biotechs specifically.
Here’s what will have happened:
- Avacta approaches multiple brokers/family offices with a too high placing price of say 90p
- More than two or three and visited and rumours spread. They shouldn’t but they do
- Risk appetite in markets still weak so nobody bites
- Forward selling
- Share price falls, then proposed placing price also has to fall
- Eventually capitulation to 50p
It’s worth noting that the last time various analysts were arguing that the company was conducting a placing, it released an RNS on 21 June 2023 stating that ‘no fundraising is imminent’ and further that it held a ‘strong cash balance of £27 million.’
There was no such update this time because they were organizing a placing. We now know that at 1 January 2024, Avacta held an unaudited cash position and principal remaining under the convertible bond amounts worth £16.6 million and £40.8 million respectively.
The only other reason there could have been for the management silence was that they were negotiating a deal with a major pharma company. This was an unlikely scenario given the stage of the assets — and therefore an objective observer would expect a placing.
It now seems likely that Avacta was attempting to organise a fundraise back in June 2023, and would have probably done a better job back then. Indeed, it had multiple chances to raise higher over the past year or so — and a competent board would have simply bit the bullet in June. Those who remember previous snap placings might be smarting.
£13 million from private investors at 95p in November 2022? No thank-you apparently. Want it half-price? Of course. And importantly, the June and January cash positions remain unaudited.
Remember that the CEO publicly called the stock overpriced not that long ago.
I went on record a couple of weeks ago noting a placing was imminent, and then tried to make a last ditch warning on a podcast with UK Investor Magazine. As expected, investors were not happy about this — but I aim to be accurate rather than popular. It’s also important to remember the names saying a raise was impossible.
The raise itself
Let’s consider the placing. Nobody cares about the breakdown, just the end result. AVCT received interest from ‘a number of high quality institutions and a European specialist healthcare fund’ and therefore decided to increase the size of the placing to £25.7 million. Directors subscribed to £65,000 of shares between them (this is nothing, especially given salaries and current holdings, and past share sales).
Bear in mind that you have the REX retail offer too. While the above placing is now worth 17.8% of the issued share capital, the retail offer will increase the dilution even further. The placing price of 50p was not only a 34% discount to the previous day’s closing price — you have to go back to the February/March 2022 crash to get a price so low.
When AVA6000 hadn’t really even got started.
CEO Alastair Smith notes that ‘under very challenging market conditions we have raised financing that allows Avacta to progress at full speed its lead pre CISION targeted chemotherapy, AVA6000, into the expansion and Phase 2 efficacy studies…this financing provides Avacta with 24 months of cash runway.’
There are two things to unpack here. The first is that while market conditions may be very challenging, the CEO has spent some time advocating that non-dilutive financing was imminent. The fact that this has not happened may suggest the assets are not as good at this stage as previously supposed — or at the very least, institutions would not lend at scale above 50p per share.
The second is that a two year cash runway derisks the company if the assets are still worthwhile. This could make buying at 50p a steal, but you need to check the changed language first, and also the tension over whether phase 2 is actually now funded by this raise.
Is AVA6000 overhyped?
We need to start with the oratory.
AVA6000 was previously a potential ‘Holy Grail’ or alternatively a ‘paradigm shift’ in oncology care.
The CEO’s words, not mine.
Now Smith notes that ‘the emerging clinical data from the Phase 1 safety study strongly supports our belief that pre|CISION can change the way in which cancer is treated and we are pleased that we are now in a position to also progress the broader pre|CISION pipeline.’
Further down, the RNS states:
‘The Directors believe that, subject to the successful completion of clinical trials and receipt of the necessary regulatory approvals, AVA6000 has the potential to compete effectively against other approaches that limit the incidence and severity of doxorubicin related toxicities.’
Moving from the Holy Grail to ‘potential to compete effectively’ is not encouraging. There is a solid chance that major pharma has told the company, in no uncertain terms, that its assets remain too early stage to be described as a potential gamechanger. The alternative is that ongoing clinical trials are less encouraging than early indications.
But perhaps more worryingly is the ‘other approaches,’ which suggests AVA6000 is not a unique approach, or that others may have better tech that may win the Dox replacement war.
Speaking of, there is no sign that the fortnightly dosing is underway. And despite previous assurances from a wide variety of investors — the reality is that the company anticipates commencing a Phase 3 trial in Q1 2026, which will need more funding and regulatory approval.
Of course, the fast-track route remains open right now, but this is another potential dagger. Indeed, both phase 2 and phase 3 are stated as subject to funding and the RNS does not note that this raise will take the flagship to the end of Phase 2 — even if it is implied by the two year cash runway. The exact phrasing is:
‘Establish recommended Phase 2 dose in Q3 2024, initiating the dose expansion phase in the US in H2 2024, followed by the Phase 2 study, subject to funding and FDA approval.’
While the fundraising has generated more than expected, investors may be feeling a distinct sense of déjà vu. The LFTs were the bees knees for some time. None were sold. Cytiva anyone? AVA6000 is now further from approval, in terms of regulation, time and money.
Turner Pope Investments notes that ‘a follow-on Phase 3 study (full design yet to be disclosed) is presently scheduled to commence in Q1 2026 and may well be conducted with a partner or partners.’ It lists Roche, Celgene, Bristol-Myers, Johnson & Johnson, Pfizer, AstraZeneca and Merck and potentials — but the previous argument was that Phase 3 would be unnecessary. And this makes clear, to me at least, that at least some analysts do not expect a partner for AVA6000 until phase 2 is over.
The question is whether this Phase 3 is simply to broaden the flagship’s reach or is needed to gain FDA approval.
However, overall and given the detail we know so far — AVA6000 and by extension preCISION remains an exceptionally promising opportunity on a scientific level. This has not changed. Reading between the lines, there are patients on the drug, who are alive right now, and without it would be long dead.
Is this price a good opportunity for new investors? Emphatically, yes.
The bottom line
When a placing is coming, the warning signs are not always there. When they are, they flash red.
The rhetoric surrounding AVA6000 has dialed down significantly. This may be for a number of reasons, but potentially is simply because it is that much further away from commercialisation. But most importantly — in the Avacta Edit, and over the past couple of years, I have repeatedly warned that even the best scientific exploratory assets are high risk, and that they require:
‘strong finances, good PR, a relatively strong share price, and decent investor sentiment to get their candidates through to commercialisation.’
The good news is that Avacta finally made a mention of a possible NASDAQ listing. But right now, even after the fundraise, the finances may not last to the end of Phase 2. It’s unclear. The PR has been abysmal. The share price is in the doldrums. And investor sentiment is at rock bottom.
The placing could have been much higher, had it been managed properly. All biotechs need someone to run the finances properly — an ex-accountant with grey hair, who wears glasses and has been surgically stripped of a sense of humour.
I do not believe that Dr Alastair Smith needs to go. He needs to be shuffled into a different role leading the therapeutic unit — which is what he’s good at. And get someone who knows the city to deal with the markets.
This article has been prepared for information purposes only by Charles Archer. It does not constitute advice, and no party accepts any liability for either accuracy or for investing decisions made using the information provided.
Further, it is not intended for distribution to, or use by, any person in any country or jurisdiction where such distribution or use would be contrary to local law or regulation.


This nails it. Well said.