Avacta is spending big on both acquisitions and R&D. But its near-term trajectory depends on the success of its proposed cancer wonder drug.

Avacta (LON: AVCT) shares have enjoyed the rollercoaster ride typical of many minor biomedical companies.
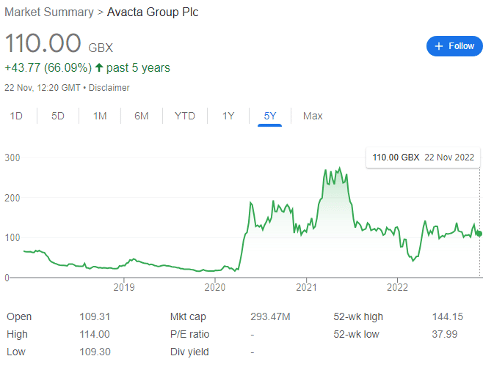
The FTSE AIM firm was swept up in the speculative frenzy of the pandemic-era lockdowns, soaring to a high of 273p by May 2021, up from just 16p pre-pandemic. However, as reality set in, Avacta’s share price corrected to 42p by early March.
But despite delivering £2.9 million in revenue last year, and seeing operating losses mount to £29 million, this could be all part of the plan. Indeed, the shares are now trading for 110p apiece, and further gains could be made soon.
As CEO Alastair Smith notes, ‘our mission is to shape the future of medicine by developing novel cancer therapies and powerful diagnostics using our proprietary Affimer and pre|CISION platforms.’
The Affimer platform has been designed as an alternative to antibodies, and eliminates the negatives of the $100 billion antibody market, including the long time taken to generate new antibodies, the human reliance on an animal’s immune response, poor specificity, and the complexity and expense of manufacture.
Meanwhile, pre|CISION is a targeted chemotherapy platform designed to only activate within fibroplast activation protein (FAP) rich tumour tissue to limit the systemic exposure that causes damage to the body’s healthy tissue.
By combining the two platforms in association with developing partnerships, Avacta has transitioned to a clinical stage biopharmaceutical company. Most importantly it’s conducting a potentially revolutionary first-in-human, dose-escalating, phase I trial of its lead pre|CISION, FAP-activated drug, code-named AVA6000, based on the generic chemotherapy doxorubicin.
Partners include globally significant biotech companies; a $400 million partnership with LG Chem, a joint venture with South Korea’s Daewoong Pharmaceutical, and a licence agreement with Point BioPharma.
Launch Diagnostics acquisition
Avacta has also conditionally agreed to purchase leading IVD distributor Launch Diagnostics for £24 million, with an earn-out capped at £13 million. The deal does make objective sense; Launch generated three-quarters of its £32.75 million in revenue in 2021 in the UK, on a gross margin within an impressive 44%-50% range.
The acquisition forms the first step in Avacta’s M&A-led strategy to build a castled position within the European immunodiagnostics market. The global IVD market is expected to reach $113.1 billion by 2026, and Launch’s non-covid contracts generate 95% repeat revenues from reliable clients such as the NHS, cancer centres, and commercial laboratories.

Smith argues that ‘this acquisition will add an established distribution channel to Avacta with three decades of customer relationships and deep market knowledge…this is a transformational moment for Avacta Diagnostics, adding a well-established route to market for existing and future in-house and acquired products.’
The capital for the acquisition is coming from a variety of sources, including a £2 million open offer and £7 million share placing, which was offered at 95p per share (4% discount). But Avacta is also taking an issue of a 6.5% £55 million 5-year, unsecured bond (convertible at 118.75p) to Heights Capital, a subsidiary of titan Susquehanna International. This represents a huge institutional vote of confidence in Avacta’s strategy.
It’s also worth noting that the fundraising effort has left Avacta with circa £40 million in cash, giving it plenty of financial headroom to acquire more complementary firms while it awaits trial results.
Where next for Avacta shares?
Avacta’s near-term future hinges on its novel AVA6000 pro-doxorubicin (chemotherapy) drug. The phase I trial began in August 2021, and by 1 September 2022, Smith was ‘very much encouraged’ by its progress, reporting that its ‘first-in-human Phase I trial (ALS-6000-101) of AVA6000 Pro-doxorubicin will advance to the fourth dose cohort of patients following a positive review of the safety and tolerability data from the dosing of the third cohort.’
The science is by its nature extremely complex, but the general idea is that the drug is only activated by fibroplast activation protein, which occurs in very high concentrations in tumour tissue compared to healthy surrounding tissue.
This means that AVA6000 could revolutionise cancer treatment by vastly reducing the undesirable side effects.
For context, anthracyclines such as doxorubicin are currently expected to grow in market value to $1.38 billion by 2024. But this estimate could skyrocket if AVA6000 becomes a standard treatment, as current drugs have limited use by virtue of their associated toxicity.
In further good news, AVA6000 was recently granted Orphan Drug Designation by the US FDA. Smith enthuses that ‘this designation provides tax credits and other incentives for drug developers addressing rare diseases. Most notably the Orphan Drug Designation will give Avacta, if AVA6000 is approved for treatment of soft tissue sarcoma, seven years of market exclusivity in the US, which is a significant commercial advantage.’
Of course, there’s a long road from phase I trial data to full licence approval. Failure could see Avacta shares consigned to the same biomedical graveyard as Synairgen, BridgeBio Pharma, Sensorion, Rafael, and Angion Biomedia.
If you haven’t heard of these companies, there’s a reason why. They all had promising initial trial results for one wonder drug, that subsequently comprehensively failed.
Of course, the rare success stories have made millionaires out of high-risk traders.
This article has been prepared for information purposes only by Charles Archer. It does not constitute advice, and no party accepts any liability for either accuracy or for investing decisions made using the information provided.
Further, it is not intended for distribution to, or use by, any person in any country or jurisdiction where such distribution or use would be contrary to local law or regulation.

Leave a comment