Avacta has expanded its footprint in the US. The listing could be next.

As a long-term investor in FTSE AIM stocks, I am used to the rollercoaster-like volatility that has been Avacta this year. Despite a strong Science Day and solid recent Phase 1 results, the biotech shares are up just 9% year-to-date.
However, it’s also up 314% over the past five years. Just look at that chart.
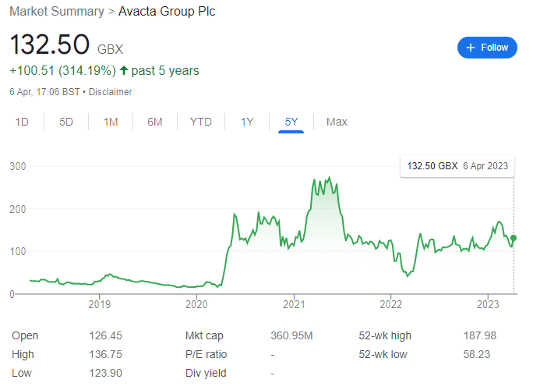
It’s so beautiful, it’s just disgusting.
Solid returns for investors, plenty of opportunity for traders. And while many find the dips annoying, I think it’s worth pointing out that the high volume is drawing increased mainstream media and institutional investor interest.
Of course, I’m relaxed — the goal is ‘chemotherapy without side effects,’ so a buyout at a huge premium could come at any time. And ever so slowly, the good news pipeline continues to deliver.
Avacta shares: recent updates
Yesterday, Avacta announced that the first patient of the fifth cohort of the flagship AVA6000 Phase 1a Dose Escalation Study has been dosed with the treatment in the UK. This ‘first-in-human phase I trial (ALS-6000-101) of AVA6000’ follows the approval of an amended clinical trial protocol by the Medical and Healthcare Products Regulatory Agency (MHRA) to allow for higher levels of dosing.
The company’s Safety Data Monitoring Committee has recommended that the trial proceed at a dosage level of at 250mg/m2 — and is aimed at identifying a maximum tolerated dose necessary to inform the dosing levels for the phase 1b, and potentially phase 2 as well.
CEO Alastair Smith enthuses that ‘we are very much encouraged by the positive safety and tolerability data emerging from the dose escalation phase 1a study of AVA6000…the recent confirmation of release of active chemotherapy in the tumour tissue and the safety data being generated in the ALS-6000-101 study are providing detailed insights.’
And today, Avacta announced that it has finally opened the first two US Clinical Investigator Sites for AVA6000 Phase 1 — sited at the Memorial Sloan Kettering Cancer Center and Fred Hutch Cancer Center.
Active enrolment has begun for soft tissue sarcoma patients in the phase 1 trial in the US, led by Dr William Tap and Dr Lee Cramer. Tap presented at the Science Day in February and is genuinely one of the world’s leading oncology experts. He would not put his name to the treatment if he didn’t think there was an excellent chance of success. And when it comes to cancer treatments, he knows more than me.
Interestingly, it seems that phase 1b will be conducted initially in the US as Avacta has claimed that the company in ‘uniquely positioned’ for further research across the pond. Avacta CDO Neil Bell notes that the development constitutes a ‘major milestone,’ and looks ‘forward to working together as we continue to build the clinical evidence base for the safety and tolerability of AVA6000, in addition to the significant tumour-targeting potential of the pre|CISION platform.’
Upcoming events and US listing?
Evidence is building that Avacta is prepping for a NASDAQ listing. I’ve called for this for some time — the AIM market simply does not have the liquidity that a company like AVCT deserves.
First, there’s this letter published by Smith in The Times earlier this year:

I’ve talked before about how the UK does little to encourage growth in the life sciences sector; but this is clearly a warning shot over the bow from the CEO. It’s fairly gratifying when someone at the top says what we’re all thinking.
Second, there’s the aforementioned opening of the US clinical sites in world-class centres, together with the linguistic hint that phase 1b may be prioritised across the Atlantic.
Third, there’s Avacta’s presentation — scheduled for 16 April — at the American Association for Cancer Research 2023 Annual Meeting in Florida. The FTSE AIM company will present a poster entitled ‘AVA3996, a novel pre|CISION medicine, targeted to the tumour microenvironment via Fibroblast Activation Protein-alpha (FAP-a) mediated cleavage.’
If AVA6000 is the flagship, AVA3996 is the lieutenant — full details are easily available on its recently refreshed site. US investors are going to want a piece of the pie.
Full-year results are being released on 25 April, and I suspect this could be the perfect opportunity to announce a NASDAQ listing.
Avacta has outgrown the AIM market.
This article has been prepared for information purposes only by Charles Archer. It does not constitute advice, and no party accepts any liability for either accuracy or for investing decisions made using the information provided.
Further, it is not intended for distribution to, or use by, any person in any country or jurisdiction where such distribution or use would be contrary to local law or regulation.

Nice summary. Not sure the nasdaq will provide the value update many investors think . Get it right and they will and should (technology needs an enormous rollout of various trails) get bought .quicker the better for patients
The NASDAQ provides greater exposure and media interest. It’s important to note that biotech or ‘life sciences’ stocks tend to become undervalued as they grow in the UK as the country lacks the infrastructure and tax regime to support phase II and phase III trials. Meanwhile, the US is far better prepared to invest in clinical stage companies — but of course, increased liquidity is a double-edged sword. One poor result and shares can fall much faster.
But at some point, this will be a required step.
Has Geoffrey Hunt responded in any way to the points raised by the CEO ofAvacta?
I would like to think that if the Government and the London stock exchange wish to slow down the migration of high growth businesses from the Uk to the USA action needs to be taken now before it is too late, otherwise it will be another missed opportunity caused by inaction especially needed now as Biden is financially encouraging business to migrate to the USA.
Jeremy hunt hasn’t directly responded, no. The recent budget is also problematic, because it reduces R&D tax credits available to early-stage UK growth stocks, hikes corporation tax, and has eradicated the previously generous super-deduction in favour of a less generous scheme.
However, for balance the LSE notes that Q1-Q3 2022 saw very robust UK biotech funding despite the high inflationary environment. The problem is that the UK isn’t good at scaling, and the public funds required to improve this (e.g. wet labs, massive subsidies) is not currently a priority.
Given the ongoing education and healthcare sector strikes, this is currently understandable, though doesn’t excuse years of prior underinvestment.